Digital contraception is a cost-effective alternative to hormonal contraception
Digital solutions challenge established perceptions of what constitutes acceptable contraceptive methods and which health benefits are considered measurable.
A newly published research article describes the treatment of patients with metastatic non–small cell lung cancer (NSCLC) in a real-world clinical setting, as well as how treatment practices have evolved since the early introduction of immunotherapy. The article was published in Acta Oncologica (Metastatic NSCLC patients in the real world in Finland).
The RWE study conducted by Medaffcon in collaboration with Bristol Myers Squibb, examined the treatment of patients with metastatic NSCLC. It illustrated how treatment practices have changed from the first years of immunotherapy to present day.
“Lung cancer is at the forefront of personalized cancer care. Treatment is advancing rapidly and has been nothing short of revolutionary. It has changed more than any other cancer type. For example, new medicines are emerging constantly. This development is described in the article,” says Sr. Scientific Advisor Riikka Mattila from Medaffcon. As part of the collaboration, the study findings provide valuable insights into how treatment practices have evolved in routine clinical care.
According to Mattila, the study is an excellent example of the importance of in-depth hospital data. Without such data, this study would have required significant manual effort. The research was based on Medaffcon’s CORE dataset, which covers data from the entire HUS region for the years 2015–2025 and was utilized in a real-world evidence project conducted in collaboration with Bristol Myers Squibb.
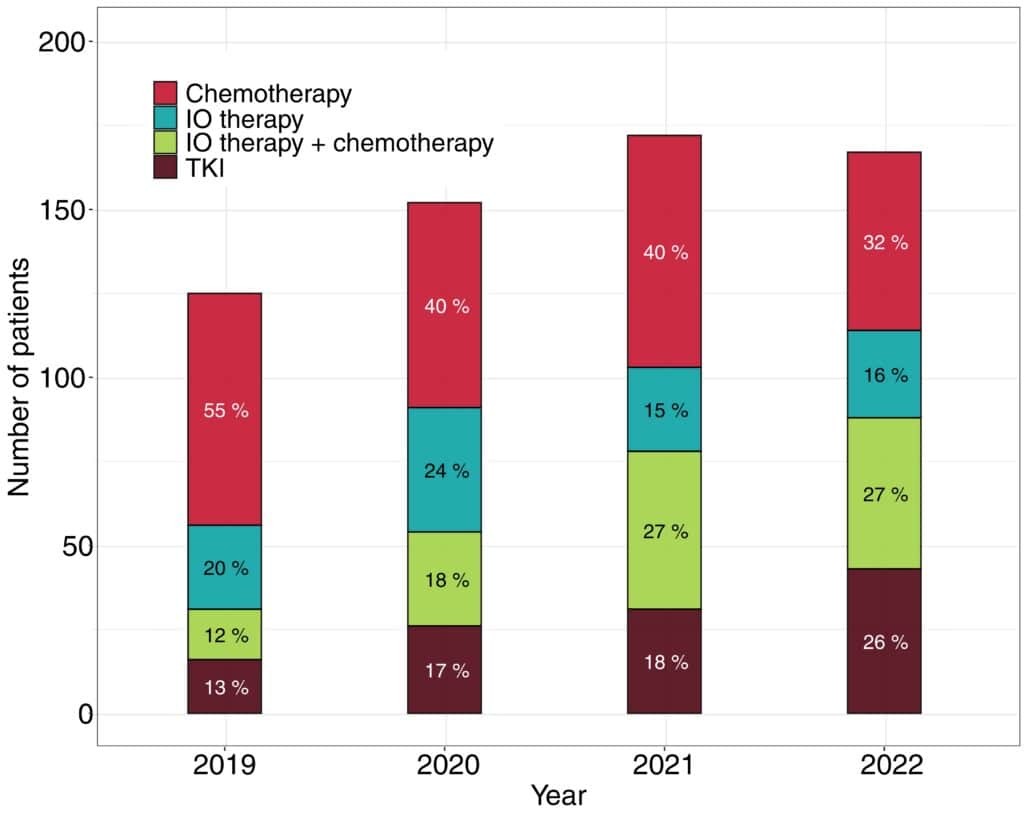
The study identified 646 patients with metastatic NSCLC. It included patients who were metastatic at diagnosis and those whose cancer later progressed to metastatic disease and who received systemic treatment. The median patient age was 70 years.
In the study, 19% of patients had squamous cell carcinoma (SqC), 68% had adenocarcinoma, and 13% had other non-SqC. Among non-SqC patients, 53% were women, whereas only 32% of SqC patients were women.
According to the article, treatments changed substantially over time, and the use of immunotherapy (IO) and tyrosine kinase inhibitors (TKIs) increased. The median overall survival was 8 months for those who received chemotherapy alone, 12 months for those treated with IO, 1 year and 2 months for those who received IO combined with chemotherapy, and 2 years for those treated with TKIs.
Mattila notes that the study also examined PD-L1 expression related to immunological treatments. Such data are not easily available in other countries, as they are not reported to quality registries. PD-L1 is essential, for example, when selecting IO-therapies for eligible patients.
According to Mattila, comparing results from different studies is challenging due to the heterogeneity of lung cancer.
Medaffcon conducted the study in collaboration with Bristol Myers Squibb, which supported and commissioned the real-world evidence project, and Helsinki University Hospital HUS, whose clinical experts contributed to the study design and interpretation.
Metastatic NSCLC patients in the real world in Finland. Ekroos H, Hölsä O, Kreutzman A, Nikkola L, Vikkula J, Mattila R, Knuuttila A. Acta Oncologica (2025)
Medaffcon Presented Insights from Finnish Real-World Data at European Lung Cancer Congress
New RWE Study Indicates Poor Prognosis for NSCLC Patients Despite Targeted Therapies

Digital solutions challenge established perceptions of what constitutes acceptable contraceptive methods and which health benefits are considered measurable.

The objective is to improve access to health data for research and innovation, but the proposal raises concerns about increased administrative burden and the role of industry within the model.

The monitoring and diagnosis of diabetic kidney disease are often insufficient in Finland. This is confirmed by two real-world evidence (RWE) studies.

Sr. Scientific Advisor
PhD
Riikka joined Medaffcon in February 2021. She has a wide-ranging experience from different therapy areas from atherosclerosis and birth asphyxia to neurodegeneration. She has more than 15 years of experience in research, three of which in Max Planck Institute in Germany. Her PhD thesis from 2011 focused on cholesterol metabolism.
Riikka’s strenghts include broad know-how and interest in diverse therapy areas, as well as enthusiasm and experience in both written and verbal scientific communication. At Medaffcon she enjoys varied projects and effectiveness of research.
Real world evidence fascinates Riikka because there is so much data, and more accumulating all the time, and most of this data is unused. There is potential for findings to support clinicians and the pharmaceutical industry, as well as alleviate the lives of patients. She is also happy that decisions in health care are increasingly evidence based.