Market Access
We support all phases of market entry and life-cycle management — from strategy and modeling to reimbursement submissions, risk sharing, interactive decision tools, and market analytics across Nordic markets.
Medicines in Finland are divided into medicines in ambulatory and hospital care. This article describes these two routes in more detail and provides information of the market access options and current pricing system in Finland.
This article is updated annually. The last update was Jan 2026.
Click a box below for more information on the route.
Medicine price components are the wholesale price, the pharmacy margin and taxes. The prices of ambulatory prescription medicines are same in every pharmacy in Finland and the medicinal decree and government act sets the statutory medicine price list, which defines the retail price based on the wholesale price (Table 1). Price formation is different for prescription and non-prescription medicines (Table 1 and 2). The government act states that the retail price for non-prescription medicines sold from community pharmacy has to be at least the pharmacy purchase price (wholesale price) and at most according to the list presented below (Table 2). The prices shown in the databases are based on price notifications submitted by pharmaceutical companies. If a particular product is not reimbursable, the pharmaceutical company can price it freely. The price of reimbursable products may not be higher than the price confirmed by the Pharmaceuticals Pricing Board.
Medicine prices are usually the retail price including value-added tax (VAT). The VAT rates are as follows: medicines, 13,5%; clinical nutrients and basic ointments when they are reimbursed, 13,5%; clinical nutrients sold without a prescription, 13,5%; basic ointments sold without prescription, 25,5%.
Prices are updated on the 1st and 15th of the month. The same prices apply in all Finnish pharmacies
When a product is dispensed on prescription, a 2,17€ dispensing fee (incl. VAT) is added to the price at the pharmacy. The price at the pharmacy is therefore higher than the one shown in the Medicinal Products Database. If the dispensed product is reimbursable, the customer is paid a reimbursement also for the dispensing fee.
Clinical nutrients and emollient creams can be sold by pharmacies also without a prescription. The price at the pharmacy will be different from that shown in the Medicinal Products Database, because the pharmacies are free to price these products.
| Prescription medicines | |
|---|---|
| Wholesale price, € | Retail price, € |
| 0 – 7,49 | 1,40 x wholesale price |
| 7,50 – 39,99 | 1,33 x wholesale price + 0,52 € |
| 40,00 – 119,99 | 1,20 x wholesale price + 5,72 € |
| 120,00 – 499,99 | 1,13 x wholesale price + 14,12 € |
| 500,00 – 1 499,99 | 1,08 x wholesale price + 39,12 € |
| 1 500 or more | 1 x wholesale price + 159,12 € |
| Non-prescription medicines | |
|---|---|
| Wholesale price, € | Retail price, € |
| 0 – 9,25 | 1,5 x wholesale price + 0,50 € |
| 9,26 – 46,25 | 1,4 x wholesale price + 1,43 € |
| 46,26 – 100,91 | 1,3 x wholesale price + 6,05 € |
| 100,92 – 420,47 | 1,2 x wholesale price + 16,15 € |
| over 420,47 | 1,125 x wholesale price + 47,68 € |
Reference:
Medicinal decree: https://www.finlex.fi/fi/laki/ajantasa/1987/19870395#P58
Government act: https://www.finlex.fi/fi/laki/ajantasa/2013/20130713
Medicinal Products Database: https://www.kela.fi/medicinal-products-database
Medicinal reimbursement system is designed to enable the acquisition of necessary medicines needed for the treatment of medical conditions at a reasonable cost. For the medicinal products that have marketing authorisation in Finland, confirmation of a reasonable wholesale price and reimbursement status are applied from the Pharmaceuticals Pricing Board (PPB, Lääkkeiden hintalautakunta, Hila), which acts under the Ministry of Social Affairs and Health, Deparment for Insurance and Social Security. The reimbursement is always tied to the accepted and authorized indication of the preparation.
Patient can receive reimbursement from the medicines, clinical nutritional supplements and basic ointments (for chronic skin disease) that a doctor has prescribed for the treatment of their disease. Thus, reimbursement can be obtained only for those medicines that are meant for the treatment of illness or relieving symptoms. Usually reimbursement is provided directly at the pharmacy during the purchase of the medicine.
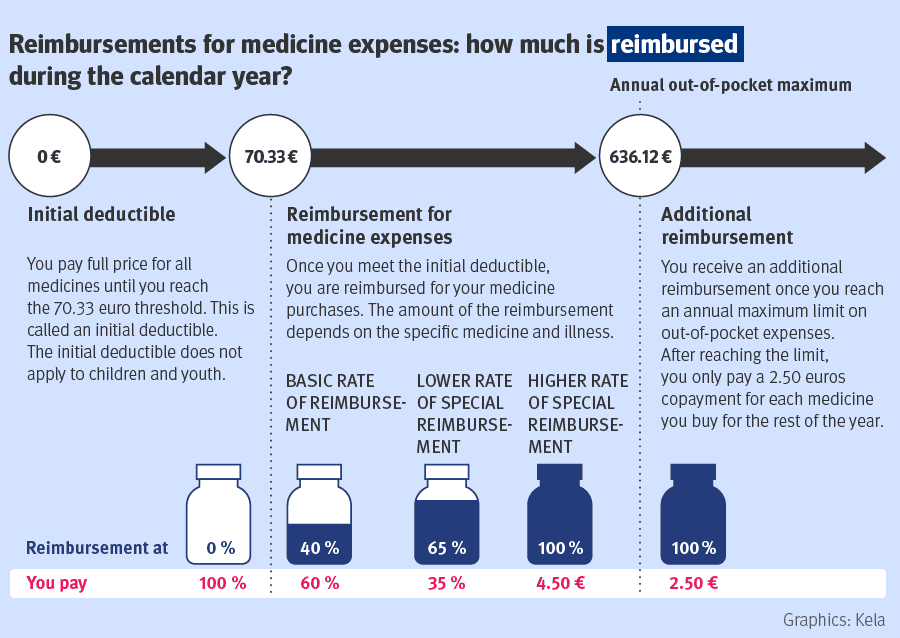
Reference: Kela, http://www.kela.fi/web/en/medicine-expenses
The patient starts receiving reimbursement after paying 70,33 € (initial deductible) of covered medicines each calendar year. Prescription medicines have three levels of compensation: basic reimbursement (40%), lower special reimbursement (65%) and higher special reimbursement (100% of the amount that exceeds co-payment 4,50€). Compensation levels are based on the severity of the disease and the necessity for medical treatment. An important part of the reimbursement system for those who have extensive medication is the maximum annual limit on medicine expenses. This means that after the medication costs during a calendar year have reached the maximum annual limit (636,12 € during the year 2026), for the rest of the year the patient pays 2,50 € for each medicine purchase.
Special reimbursement for a medicinal product may be granted for severe and long-term illnesses as defined in the Government Decree. A prerequisite for special reimbursement is that the medicine first obtains basic reimbursement status. When deciding on special reimbursement status, the PPB will consider for example the type of the disease as well as the therapeutic necessity and value of the product in the treatment of this severe and long-term illness, the replacement or remedial mode of action of the product in addition to the the cost-effectiveness and expected costs for the reimbursement system resulting from the applied special reimbursement. The medicinal product can be approved for special reimbursement when there is sufficient experience of use and research evidence to support the application.
The decision on the special reimbursement of a medicinal product may also be limited only to a particular form or degree of illness.
New drugs for unmet medical needs are constantly entering the market. Typical for these types of drugs are high price, a targeted / small patient group and limited amount of knowledge available regarding total costs, cost-effectiveness and/or therapeutic value of these new medicines. The goal of conditional reimbursement aims to control these uncertainties through a confidential agreement process. New pharmacotherapy is the primary group eligible for conditional reimbursement (new active substances and extensions to new significant therapeutic indications).
After Marketing Authorisation holder (MAH) has given a proposal for conditional reimbursement and the PPB considers the product eligible, negotiations are initiated between the PPB and the MAH. If conditional reimbursement is confirmed by the PPB the decision is attached with a confidential agreement between the PPB and the MAH detailing the conditions for monitoring and controlling the uncertainty associated with the medicinal product. So far, all agreements have been financial agreements with payback arrangements to Social Insurance Institution’s Health Insurance Fund in accordance with the criteria specified in the agreement [1]. The Social Insurance Institution is responsible for the implementation.
As with all decisions by the PPB regarding reasonable wholesale price and reimbursement status of a product, the conditional reimbursement status is valid for a fixed period of time. During the validity period the eligibility of a product for conditional reimbursement will be reassessed if the MAH applies for a renewal or extension of the product’s conditional reimbursement status or for special reimbursement status. These applications should already at the time of submission include as a separate document a proposal for continuation of the conditional reimbursement status describing the changes that have happened and the effects they will have on the grounds for a conditional reimbursement status and on the proposed measures to control uncertainty.
Reference:
[1] Martikainen J, Pelkonen L: Lääkkeiden ehdollinen korvattavuus ja järkevä lääkehoito. Lääketieteellinen aikakauskirja Duodecim, 2020;136(2):200-205.
Pharmaceuticals Pricing Board – https://www.hila.fi/en/
Medicines intended primarily for use in hospital care follow a distinct market access pathway in Finland compared with outpatient medicines. Hospital medicines are typically high-cost, specialised therapies used in secondary or tertiary care, and their introduction is guided by structured assessments of clinical value, cost-effectiveness and budget impact.
In Finland, access to hospital medicines is organised through two complementary assessment routes: a national HTA and recommendation process coordinated by Fimea and COHERE Finland, and hospital-based HTA conducted locally by hospital districts and university hospitals. Regardless of the route, procurement through tendering ultimately determines routine availability in hospitals.
The primary national route for hospital medicines is the health technology assessment (HTA) and recommendation process coordinated by Fimea and COHERE Finland. This route is most commonly used for new, high-impact hospital medicines with significant clinical or economic implications for the healthcare system.
Following marketing authorisation, Fimea performs an HTA focusing on:
The HTA may be initiated by Fimea or conducted with company involvement through a structured submission process. The resulting assessment report forms the evidence base for deliberation at COHERE Finland.
COHERE Finland issues a national recommendation on the use of the medicine in hospital care. Recommendations may be positive, conditional, or negative, and they are intended to support consistent and equitable decision-making across hospital districts. While the recommendation itself is not legally binding, it strongly guides hospital uptake, price negotiations and procurement decisions.
If COHERE issues a conditional recommendation that requires price moderation, the medicine may proceed to national price negotiations. Acting on behalf of all the university hospitals, HUS Pharmacy contacts the pharmaceutical company and initiates negotiations. The process is not a tender or auction; negotiations are confidential and usually take place in several rounds until a target price is reached or the parties decide not to proceed.
In parallel with the national process, hospital medicines may enter use through hospital-based HTA conducted at the local or regional level. Individual hospital districts or university hospitals can assess new medicines independently, particularly when:
Hospital-based HTA typically evaluates clinical benefit, comparators, and the expected local budget impact. Decisions are made by hospital-level expert bodies, such as pharmacy and therapeutics committees, and lead to local formulary inclusion and procurement within that hospital district.
This route enables earlier or more targeted access, but decisions apply only to the assessing hospital(s) and do not create national alignment.
Confidential agreements, including risk-sharing and managed entry agreements, are an increasingly important enabling mechanism in hospital market access. They are not a separate access route, but rather a tool used to address uncertainties identified during national or hospital-based HTA.
Such agreements may be applied when:
Confidential agreements are commonly negotiated following a conditional COHERE recommendation or in connection with hospital-based HTA decisions, and they often facilitate broader or faster access to high-cost hospital medicines.
In Finnish hospital care, procurement through competitive tendering is the final step that translates assessments and recommendations into routine clinical use. Even with a positive national recommendation or favourable hospital-based HTA outcome, medicines must be included in procurement contracts to become widely available.
All hospitals in Finland and their outpatient clinics use medicines intended for hospital use or those also indicated for outpatient use. Hospitals procure all their medicines through competitive bidding. Hospital sales account for about one fourth of the Finnish pharmaceutical market (PIF). Hospitalized patients get medicines free of charge.
The Wellbeing Service Counties who are owners of hospitals are covered by the competitive procurement obligation by law. The Act on Public Contracts sets strict terms for the procedures to be followed by the public sector (Laki julkisista hankinnoista ja käyttöoikeussopimuksista, 1397/2016).
Tendering may be organised at:
and typically includes price competition, contract conditions, and supply requirements. Tendering ensures cost control and efficient use of public resources, and it ultimately determines which medicines are purchased, at what price, and under which conditions.
Fimea: Assessment of hospital-only medicinal products
Fimea’s page confirming hospital medicines assessment focus and application timing
Fimea: Assessment of pharmacotherapies (HTA) – explains Fimea’s role in therapeutic and economic assessments of medicines
Ministry of Social Affairs and Health (STM): Legislation, steering and supervision, cooperation — describes the role of COHERE Finland in service choices & recommendations based on Fimea assessments
COHERE Finland (service choices in healthcare) — background on how the Council issues recommendations
For HUS or other districts, individual hospital HTA info often appears on hospital sites or internal documents; the general HUS pharmacy page indicates procurement and tendering roles.
Medaffcon article on national price negotiation of hospital-only medicines — explains how conditional recommendations from COHERE can lead to price negotiation
EU-Healthcare overview of service choices context in Finland
All Medaffcon Market Access articles – Find all our market access related articles here.

We support all phases of market entry and life-cycle management — from strategy and modeling to reimbursement submissions, risk sharing, interactive decision tools, and market analytics across Nordic markets.
We build rigorous cost-effectiveness and budget impact models, integrating real-world data and assumptions to inform reimbursement decisions, payer negotiations, and strategic planning.
We guide you through the full reimbursement journey — strategic counsel, dossier crafting, submission, defense, and linkage to evidence generation to secure access under optimal conditions.
We deliver a reporting tool tracking patient flows and reimbursement data for outpatient medications, enabling sales, marketing, and management to monitor brand performance and market trends.
We transform complex clinical, economic, and utilization data into intuitive, stakeholder-facing tools (dashboards, calculators) to support internal decisions, payer dialogue, and external communication.
We design and negotiate performance-based or conditional reimbursement contracts, structuring pricing, evaluation metrics, and risk allocation to accelerate access and balance stakeholder interests.
We supply experienced interim professionals to fill gaps in medical or market access teams, manage deliverables, and ensure continuity during organizational transitions.
We design and deliver tailored training and coaching programs for medical, market access, commercial, and cross-functional teams — enhancing scientific knowledge, communication skills, and stakeholder engagement capabilities.

Medaffcon develops the Patient Dynamics tool based on customer feedback and user experience.

Patient Dynamics tool compiles up-to-date and regional data on medicine use in Finland and Sweden.

The aim of the cooperation is to harmonize processes and produce joint assessments that each country can use in its own decision-making.

Market Access Lead
MSc (Health Sciences), BBA
+358 40 580 0567
simo.jaaskelainen@medaffcon.com
Simo started as the leader of Medaffcon’s Market Access team in November 2024. He is a returnee, as he has also previously worked at Medaffcon. Before returning to Medaffcon, Simo worked in a pharmaceutical company as the head of a Market Access team and has also gained Market Access experience in other pharmaceutical companies. He has over 20 years of experience in the pharmaceutical industry. Simo holds a Master’s degree in Health Sciences and a Bachelor’s degree in Business Administration.
Simo brings to Medaffcon a strong and versatile background in the pharmaceutical industry, particularly in Market Access roles. His strengths include versatility, organizational skills, and the ability to see the bigger picture. In the field of Market Access, Simo does not consider himself an expert in any particular subfield but sees himself as a generalist. He is drawn to the challenges of the industry and the opportunity to find comprehensive solutions that meet the needs of clients.
Ongoing changes in the operating environment require pharmaceutical companies to adapt and adopt new ways of working. Simo believes that the role and importance of Market Access will become even more prominent in a situation where society’s willingness to pay and the needs of the pharmaceutical industry must be aligned in a way that benefits both parties.